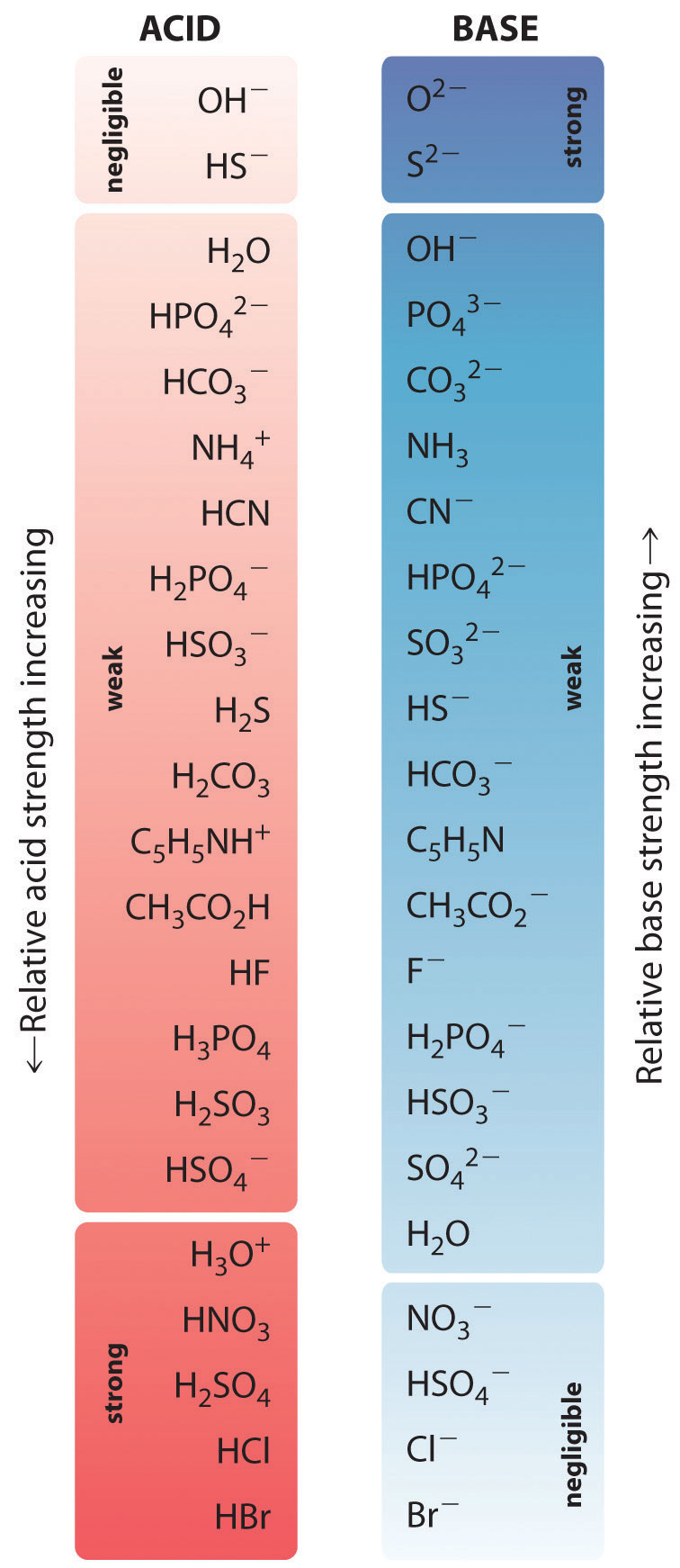
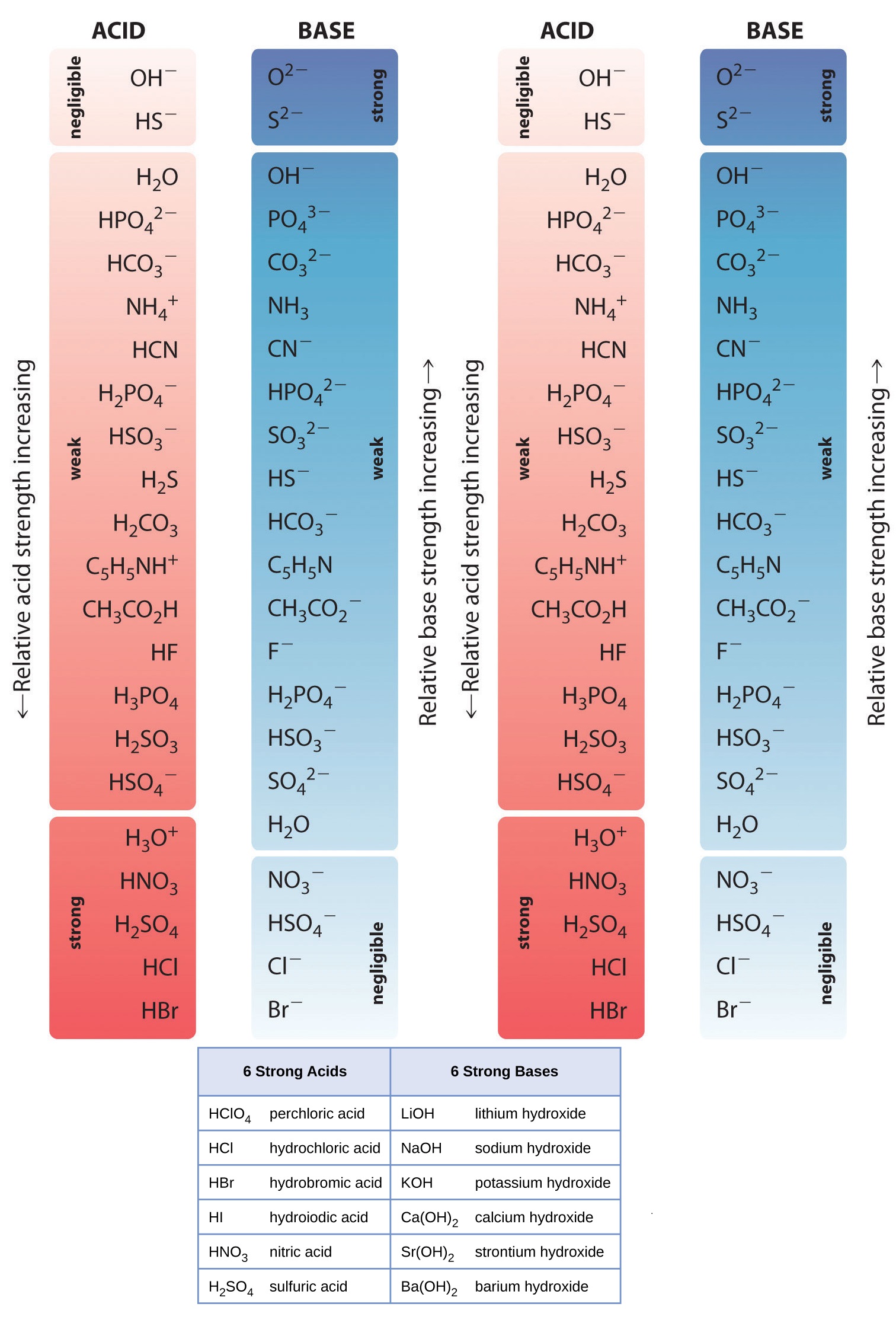
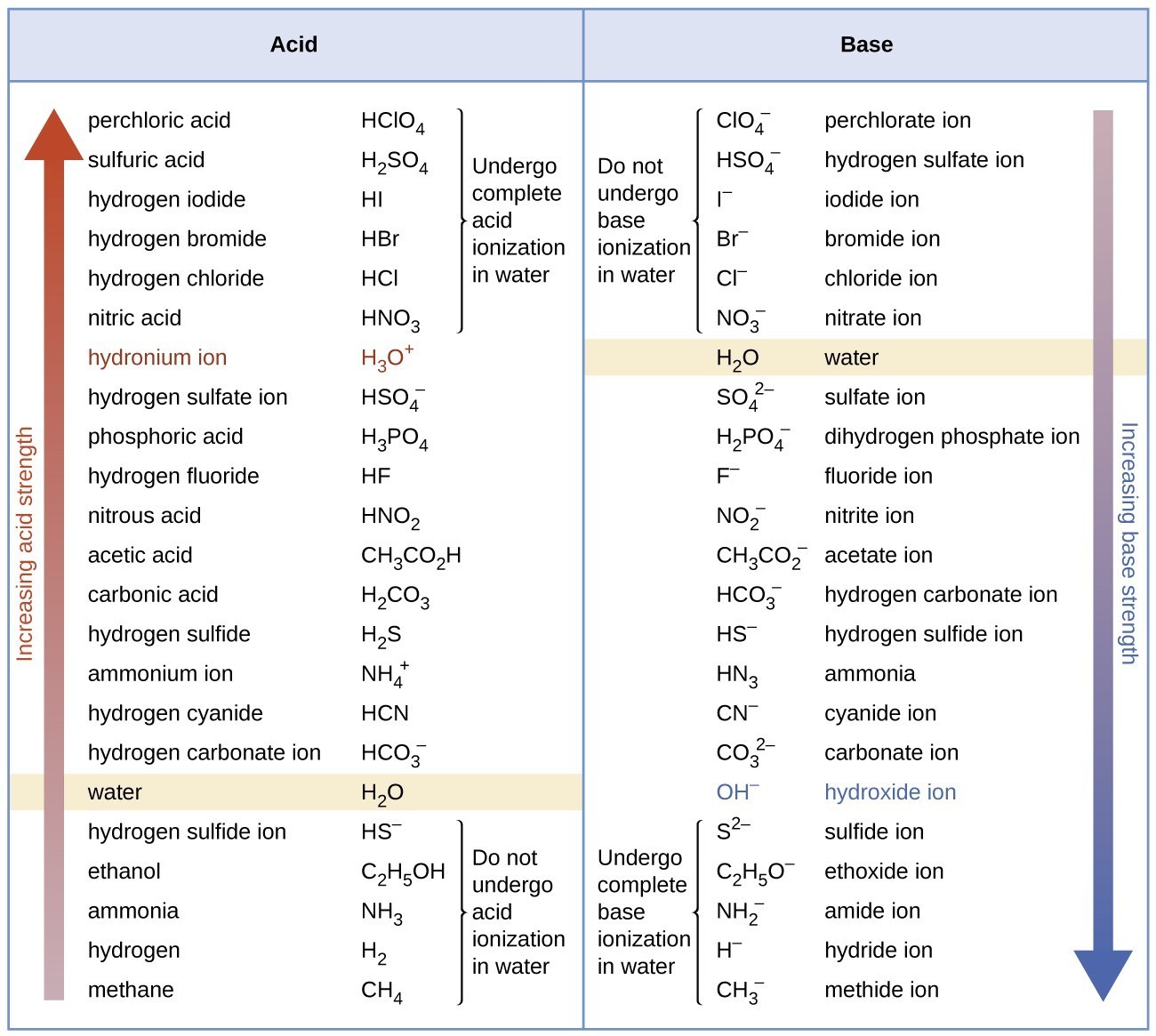
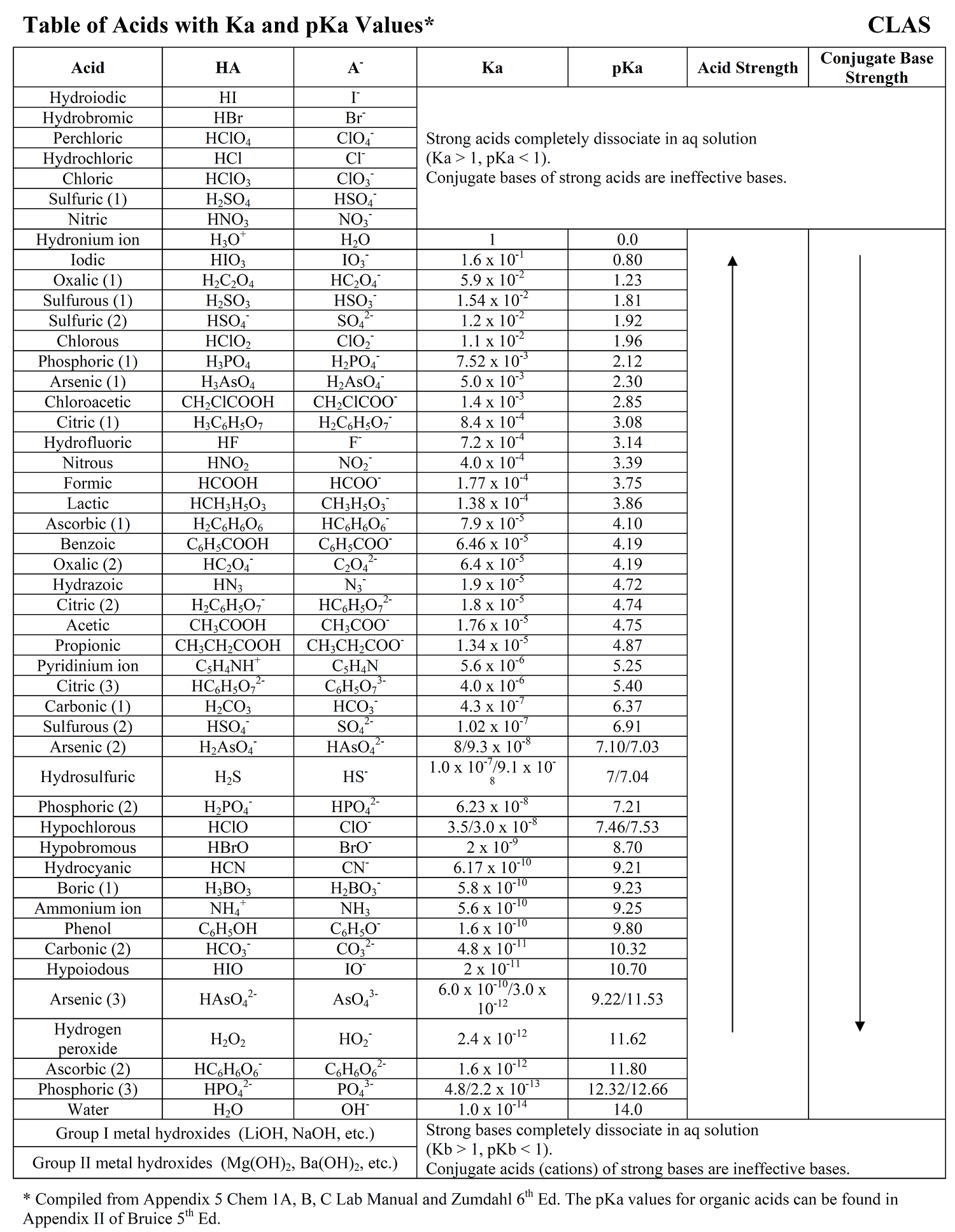
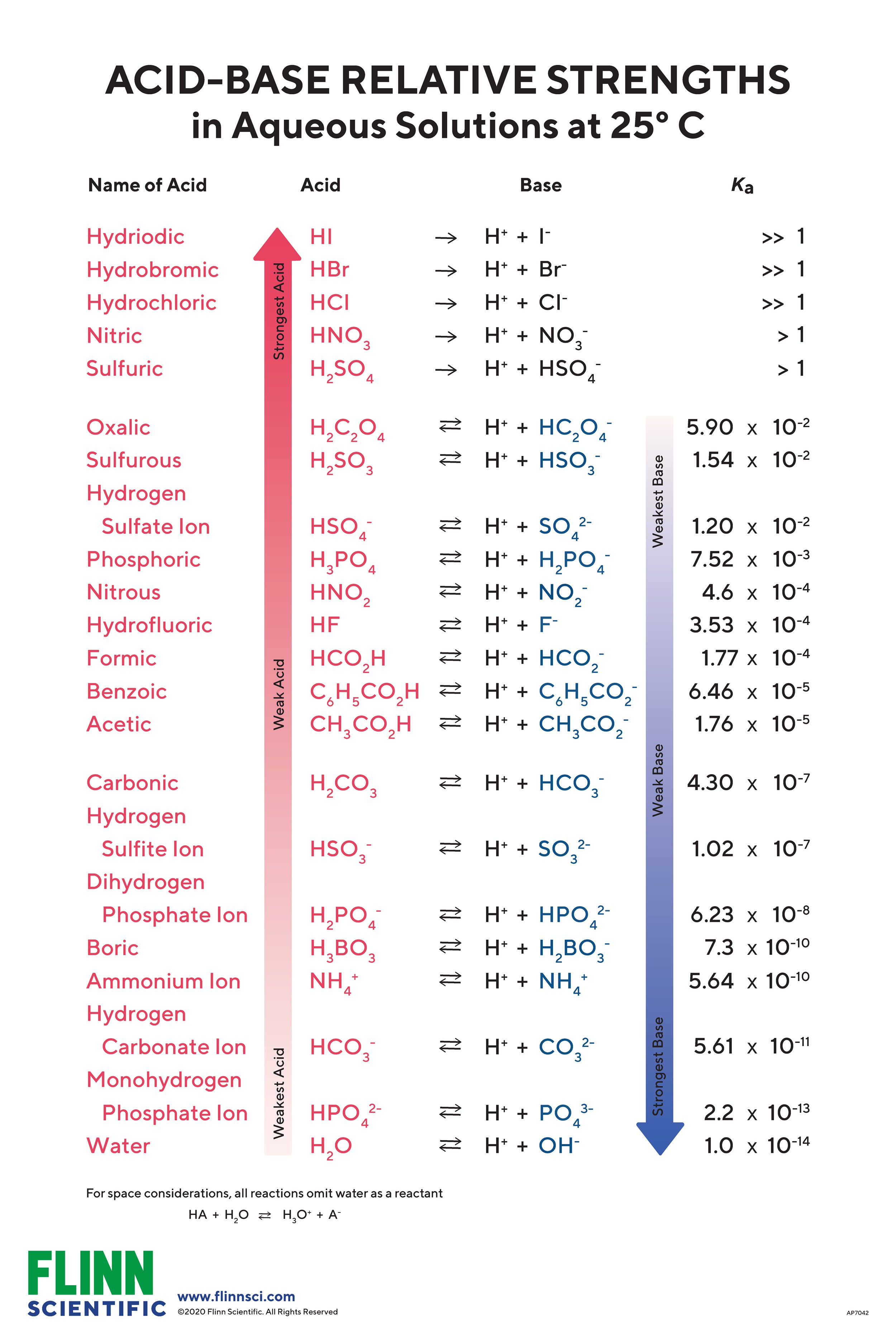
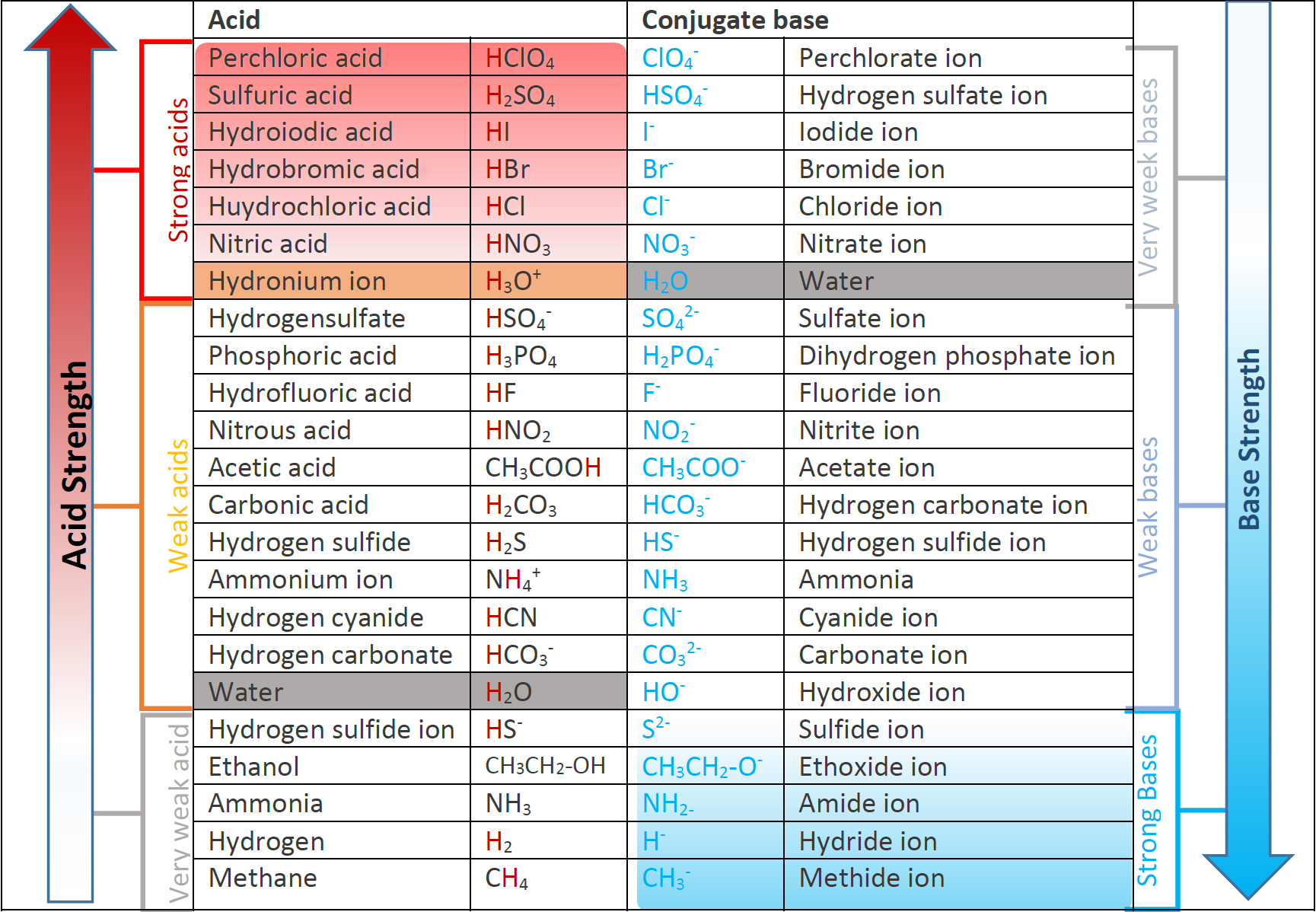
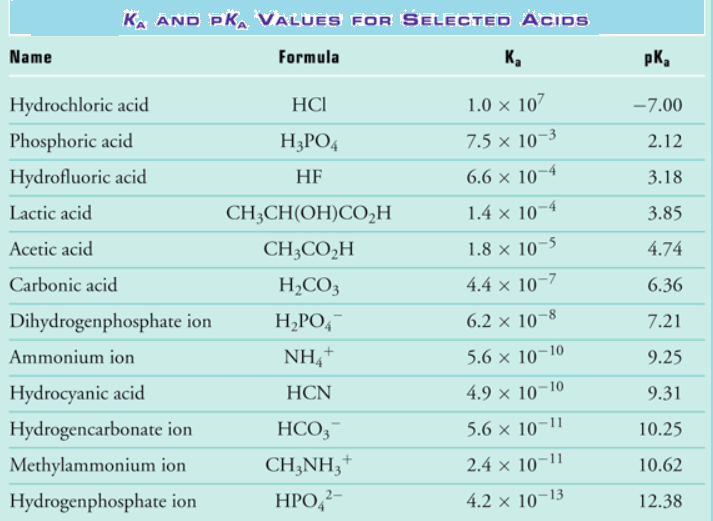
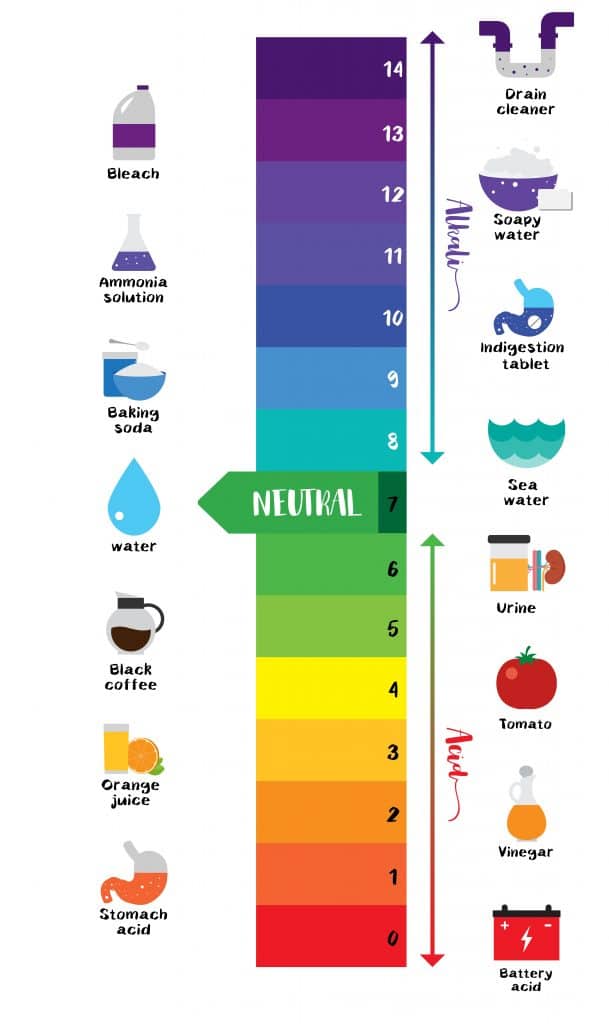
Acid Strength Chart
Acid Strength Chart - The first six acids in figure 14.3.3 are the most common strong acids. Acid strength increases down a group and increases from left to right across a period. A stronger acid (larger ka) has a smaller p ka, and a. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Chart or notebook size available. If relatively little ionization occurs, the acid or base is weak. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. (select option to see volume pricing availability) 8½ × 11, pad of 30 (ap7229) $24.50. Web definitions of ph, poh, and the ph scale. They’re routinely described as strong or weak, concentrated or dilute. A stronger acid (larger ka) has a smaller p ka, and a. 48 × 32, each (ap7042). A guide to acids, acid strength, and concentration. Web chart of acid and base strength. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. By andy brunning september 28, 2016. Chart or notebook size available. Web acid with values less than one are considered weak. Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. The relationship between acid strength and the ph of a solution. Web definitions of ph, poh, and the ph scale. Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. For each acid, the ionization reaction shows the acid’s conjugate base. The strong bases are listed at the bottom right. By andy brunning september 28, 2016. Web the strength of acids and bases, i.e., the extent of dissociation of the dissolved acid or base into ions in water is described. Calculating the ph of a strong acid or base solution. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Web chart of acid. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. The relationship between acid strength and the ph of a solution. Web the strength of acids and bases, i.e., the extent. Web use this acids and bases chart to find the relative strength of the most common acids and bases. Pka = −log ka p k a = − log k a. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. If the ionization reaction is essentially complete, the acid or base is termed. If the acid or base conducts electricity strongly, it is a strong acid or base. By andy brunning september 28, 2016. The relative strength of the acid/conjugate base pair is also. The relationship between acid strength and the ph of a solution. Web acid with values less than one are considered weak. Web definitions of ph, poh, and the ph scale. Web figure 15.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. They’re routinely described as strong or weak, concentrated or dilute. Web use this acids and bases chart to find the relative strength of the. Web acid with values less than one are considered weak. If the acid or base conducts electricity strongly, it is a strong acid or base. Web the key to understanding this trend is to consider the hypothetical conjugate base in each case: If relatively little ionization occurs, the acid or base is weak. (select option to see volume pricing availability). If the acid or base conducts electricity strongly, it is a strong acid or base. Look at where the negative charge ends up in each conjugate base. Web acid strength is the tendency of an acid, symbolised by the chemical formula, to dissociate into a proton, +, and an anion,. Web the terms strong and weak give an indication of. The first six acids in figure 14.3.3 are the most common strong acids. Web acid strengths are normally expressed using p ka values rather than ka values, where the pka is the negative common logarithm of the ka: Web a strong acid yields 100% (or very nearly so) of \(\ce{h3o+}\) and \(\ce{a^{−}}\) when the acid ionizes in water. They’re routinely. The strength of acids and bases is determined by their ability to donate or accept protons (h ions) and can be quantified using the concepts of dissociation in water and the corresponding equilibrium constants. The terms strong and weak describe the ability of acid and base solutions to conduct electricity. Web figure 15.3.3 lists a series of acids and bases. By andy brunning september 28, 2016. This information can be used to predict the outcome of reactions between acids and other substances, such as bases and metals. Web figure 15.3.3 lists a series of acids and bases in order of the decreasing strengths of the acids and the corresponding increasing strengths of the bases. If relatively little ionization occurs, the acid or base is weak. Web determine at a glance the relative strengths of a host of acids and bases. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions. Web the strength of acids and bases, i.e., the extent of dissociation of the dissolved acid or base into ions in water is described. The first six acids in figure 15.3.3 are the most common strong acids. 48 × 32, each (ap7042). Web acid strengths are normally expressed using p ka values rather than ka values, where the pka is the negative common logarithm of the ka: If the ionization reaction is essentially complete, the acid or base is termed strong; Web the relative strength of an acid or base is the extent to which it ionizes when dissolved in water. Even if you’re not a chemist, you’ll doubtless remember learning about acids back in school. Web the terms strong and weak give an indication of the strength of an acid or base. The first six acids in figure 14.3.3 are the most common strong acids. The terms strong and weak describe the ability of acid and base solutions to conduct electricity.14.3 Relative Strengths of Acids and Bases Chemistry LibreTexts
Acid Strengths Table
List of Strong Acids & Bases in Order StudyPK
Relative Strengths of Acids and Bases Chemistry Atoms First
Acid Strength, Ka, and pKa Chemistry Steps
AcidBase Strength Charts for Chemistry
6.3 Strength of acids and bases Chemistry LibreTexts
Acid strength W3schools
Section 3 Strengths of Acids and Bases Nitty Gritty Science
pKa Values and strengths of Acids and Bases
Web Use This Acids And Bases Chart To Find The Relative Strength Of The Most Common Acids And Bases.
If The Ionization Reaction Is Essentially Complete, The Acid Or Base Is Termed Strong ;
Table \(\Pageindex{1}\) Lists Several Strong Acids.
Calculating The Ph Of A Strong Acid Or Base Solution.
Related Post: